Pyrolysis of plastic to diesel is a chemical recycling process. It converts plastic waste into a usable diesel-like fuel through thermal decomposition under oxygen-free conditions. It offers a potential solution for managing non-recyclable plastic waste and creating value from pollution.
Not all plastics can be converted into high-quality fuel oil. The ideal feedstock is polyolefins, which make up a large portion of plastic waste.
High-quality feedstocks for pyrolysis of plastic to diesel include PE, PP, and PS.
- PE (Polyethylene): High-density polyethylene (milk jugs, detergent bottles) and low-density polyethylene (plastic bags, film).
- PP (Polypropylene): Food containers, yogurt tubs, bottle caps.
- PS (Polystyrene): Foam cups, packaging foam.

Unsuitable feedstocks for plastic pyrolysis oil:
- PET (Polyethylene terephthalate): Soda bottles. It releases oxygen, forming acidic compounds (such as benzoic acid), which corrode equipment and contaminate the oil.
- PVC (Polyvinyl chloride): It releases hydrochloric acid (HCl) gas, which is highly corrosive and toxic. PVC also contaminates the oil with chlorine, rendering the fuel unusable without extensive and expensive cleaning. Pretreatment to remove PVC is crucial.
- Nylon and polyurethane (PU): Contain nitrogen and oxygen, which can cause oil contamination and instability.
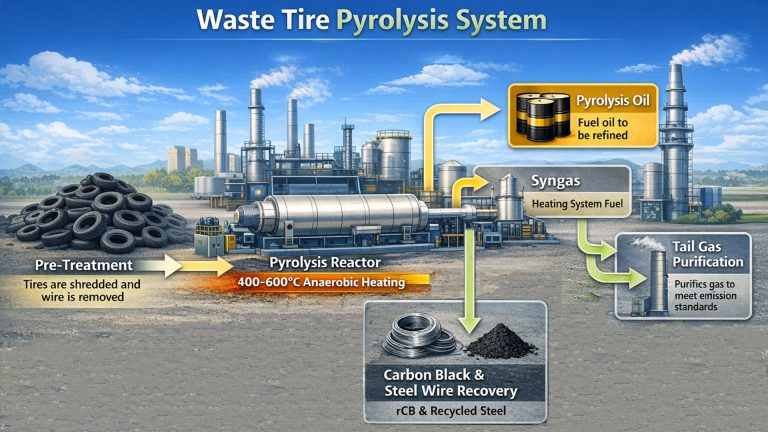
Pyrolysis of Plastic to Diesel Process
Pre-treatment
Pretreatment is crucial for removing contaminants and non-plastic materials, especially polyvinyl chloride (PVC) and polyethylene terephthalate (PET). This is a crucial step that directly determines the entire pyrolysis of plastic to diesel process.
Sorting: This is crucial. Plastics unsuitable for pyrolysis of plastic to diesel, such as PVC and PET, must be removed.
Shredding: Breaking large plastic pieces into smaller pieces. This increases the heating surface area and ensures a more uniform reaction.
Washing: Removes impurities such as sand, oil, and labels from the plastic surface. These impurities can affect the oil and create residue.
Feeding
Pretreated plastic is fed into the high-temperature plastic pyrolysis equipment via a closed feeding system (screw feeder). Air ingress is minimized throughout the entire feeding process.

Pyrolysis Reaction
This is the core conversion step in the pyrolysis of plastic to diesel process. The reactor is heated to a specific temperature between 350°C and 500°C (temperature control is crucial).
In the absence of oxygen, the plastic’s molecular chains undergo random cleavage (pyrolysis). Long-chain polymers are broken down into smaller molecules, transforming from a solid to a gaseous state. The reactor is typically equipped with an agitator to ensure uniform heating.
Condensation
The resulting high-temperature oil-gas mixture enters a condensation system (typically a multi-stage condenser) from the reactor. Condensable gases are cooled by a coolant (usually water) and condensed into liquid crude oil. Non-condensable gases (such as methane, ethane, propane, and ethylene) are then processed further.
Product Handling and Refining
Crude Oil (Plastic Pyrolysis Oil)
The crude oil obtained upon condensation is a dark brown mixture with a pungent odor. Its composition is complex and its stability is poor, making it unsuitable for direct use as diesel. It requires further distillation refining to separate its components into light naphtha, diesel fraction, and heavy oil based on their boiling points.
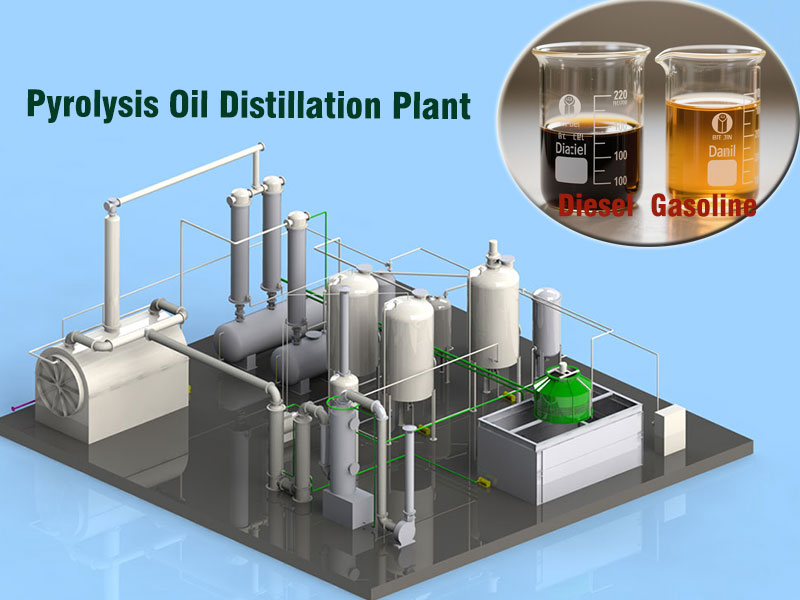
Pyrolysis oil is a complex mixture containing a variety of components, ranging from gasoline to diesel and heavy oil. Its separation and purification are key steps in pyrolysis of plastic to diesel process.
First, pyrolysis oil is initially separated into light fractions (such as gasoline), middle fractions (diesel), and heavy fractions (heavy oil) through distillation equipment, based on the boiling point differences of these fractions. Precise control of the distillation temperature and reflux ratio is crucial during the distillation process.
Generally, the boiling point of the diesel fraction ranges from 180°C to 360°C. By adjusting the temperature of the distillation column, the diesel fraction vaporizes and condenses within this temperature range for collection.
The diesel fraction obtained after initial separation still contains impurities, such as heteroatom compounds like sulfur, nitrogen, and oxygen, as well as incompletely cracked large hydrocarbons. These impurities can affect the quality and performance of the diesel, necessitating further purification.
Common purification methods include acid washing, alkaline washing, and adsorption refining.
Acid washing, typically using dilute sulfuric acid or phosphoric acid solutions, removes basic nitrogen compounds and some sulfides from diesel fuel. Alkaline washing, using sodium hydroxide solution, neutralizes acidic impurities. Adsorption refining utilizes adsorbents, such as activated clay, to remove pigments, colloids, and residual impurities from diesel fuel, improving its purity and stability.

After these purification steps, pyrolysis of plastic to diesel quality can be significantly improved, reaching or approaching the quality standards of conventional diesel fuel.
Non-condensable Gas
These gases are typically recovered to provide fuel for pyrolysis reactor heating. This makes the system energy self-sustaining and reduces external energy consumption.
Solid Residue (Carbon Black)
The solid residue remaining after the reaction is primarily carbon black, along with some inorganic impurities. This can be burned as solid fuel or processed for industrial use as carbon black.