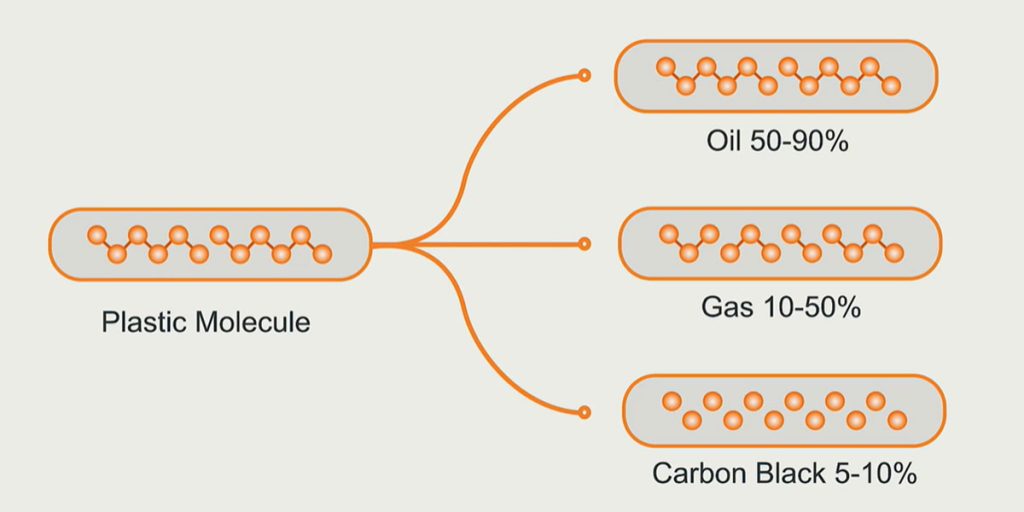
The plastic pyrolysis process uses chemical recycling to process waste plastics. The main components of waste plastics are high molecular polymers (polyethylene PE, polypropylene PP, polystyrene PS). Plastic pyrolysis plant decomposes plastic waste thermally under oxygen-free conditions, converting it into valuable products such as fuel oil, syngas, and carbon black.
To address the plastic waste problem, various methods have been explored, including landfilling, incineration, and mechanical recycling.
While landfill disposal is simple and easy, it consumes significant land resources. Furthermore, plastic waste persists in landfills and can produce hazardous substances, polluting soil and groundwater.
Incineration can effectively reduce the volume of plastic waste, but the process produces large amounts of harmful gases, such as dioxins and furans. These gases seriously affect air quality and harm human health.
While mechanical recycling can reprocess some plastics, it is limited by factors such as the type of plastic and impurities. The quality of recycled plastic is often inferior to virgin plastic, and it can only be recycled a limited number of times. Ultimately, a large amount of unrecyclable plastic waste is generated.

The pyrolysis technology for plastic recycling offers a new approach and solution to the plastic waste problem, becoming a novel plastic waste treatment technology. Plastic pyrolysis is a chemical recycling technology that converts waste plastics into small-molecule chemicals through a high-temperature, anaerobic, or anoxic thermal decomposition reaction. Unlike mechanical recycling, it can process mixed, heavily contaminated, or difficult-to-process plastics, making it an important supplementary means of addressing “white pollution.”
Pyrolysis Chemical Recycling
Plastic pyrolysis refers to the process of using heat in an oxygen-free or low-oxygen environment to break the chemical bonds within long-chain plastic molecules, allowing them to recombine into smaller compounds. The plastic pyrolysis process is like breaking a long, complex molecular chain into smaller components, which are then reassembled into new, simpler structures.
At a molecular level, plastics are composed of polymers. These polymers are composed of a large number of repeating units linked by covalent bonds, forming a long, chain-like molecular structure.
Take common polyethylene (PE) pyrolysis as an example. It is made from the monomer ethylene, and its molecular structure contains numerous carbon-carbon (C-C) and carbon-hydrogen (C-H) bonds. During the pyrolysis process, when sufficient heat is applied, these chemical bonds absorb energy, become unstable, and eventually break.
C-C bond cleavage is a key step in the plastic pyrolysis process, as it directly leads to the decomposition of the polymer. As C-C bonds break, the long-chain molecules are broken into shorter fragments. These fragments further react to produce various small-molecule compounds, such as hydrocarbons, hydrogen, and carbon monoxide.
Plastic Pyrolysis Process
The plastic pyrolysis process can be roughly divided into three stages, each accompanied by distinct physical and chemical changes.
The drying stage primarily removes moisture and low-boiling-point impurities from the plastic. This stage is crucial, as moisture can interfere with the pyrolysis reaction, potentially leading to energy waste and reduced product quality.
As the temperature gradually rises, the pyrolysis stage begins, where high-molecular-weight polymers begin to undergo cracking reactions. As the temperature rises further, the pyrolysis reaction intensifies, producing a greater variety and quantity of small-molecule products.
During the waste plastic pyrolysis process, different types of plastics exhibit varying pyrolysis behavior and product distribution. Polyethylene primarily produces various hydrocarbon compounds during pyrolysis, including alkanes and alkenes. The carbon chain length and degree of saturation of these hydrocarbons vary with pyrolysis conditions.
Finally, the cooling stage cools the high-temperature gaseous products produced by pyrolysis. Some of these gaseous substances condense into liquid, forming pyrolysis oil. The uncondensed gases become pyrolysis gas.


Factors Affecting the Plastic Pyrolysis Process
During the plastic pyrolysis process, parameters of plastic pyrolysis plant have a significant impact on the composition and properties of the plastic pyrolysis products, such as temperature, pressure, and reaction time.
Temperature is a key factor influencing pyrolysis products. At lower temperatures, the plastic pyrolysis reaction proceeds more slowly, and the products may contain more macromolecular substances, such as waxy solids.
As the temperature increases, the pyrolysis reaction rate accelerates, and the proportion of small molecular products increases. When the temperature exceeds 600°C, the main pyrolysis products are mixed fuel gases, such as light hydrocarbons like methane (CH₄) and ethylene (C₂H₄). These gases have high calorific value and can be used as fuel.
At temperatures between 400°C and 600°C, the main cracking products are mixed light hydrocarbons, naphtha, heavy oil, kerosene, and waxy solids.
The optimal pyrolysis temperature varies for different types of plastics. The pyrolysis temperature of polyethylene is generally around 450-550°C, while that of polypropylene is slightly higher, around 500-600°C.
Pressure also has a certain impact on the pyrolysis reaction. Appropriately reducing the pressure can promote the formation of small molecule products. This is because a low-pressure environment facilitates the escape of gaseous products, thus driving the reaction toward the formation of small molecules.
Reaction time is also important for plastic pyrolysis process. If the pyrolysis time is too short, the plastic may not be fully decomposed, resulting in a high content of unreacted raw materials in the product. If the pyrolysis time is too long, secondary reactions may occur, causing further decomposition or polymerization of the products, affecting product quality and yield.