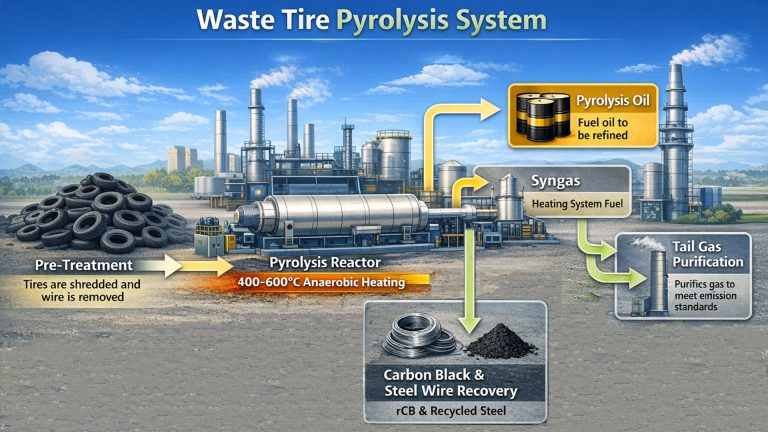
Pyrolysis technology provides an important way for solid waste resource utilization and energy recovery. Waste pyrolysis is particularly suitable for treating solid waste rich in organic matter (such as waste plastics, waste rubber, biomass waste, sludge, etc.)
Pyrolysis is the most effective method for recycling organic waste. This method involves continuously breaking down organic macromolecular chains in an oxygen-free environment, ultimately converting them into smaller molecules. Ultimately, these products yield usable products such as pyrolysis oil, carbon black, and pyrolysis gas.
Through pyrolysis of solid waste, the organic matter in the waste is broken down into smaller molecules, thereby reducing the volume of the waste. At the same time, the gases produced during the pyrolysis process are purified to meet environmental standards, achieving waste reduction and harmlessness.


Differences in Rubber and Plastic Waste Pyrolysis
Researchers have processed plastic and rubber waste from landfills through solid waste pyrolysis. They found that rubber pyrolysis can produce significant amounts of charcoal, while plastics have value in producing pyrolysis oil. By adjusting pyrolysis parameters, they were able to selectively target pyrolysis products. This waste pyrolysis treatment is not only environmentally friendly and reduces pollution, but also unlocks the potential value of organic waste.
Different organic wastes require different pyrolysis methods. To improve efficiency, research on the pyrolysis of organic waste is necessary, and adopting appropriate research methods is crucial.
Differences in Pyrolysis Principles
Both rubber and plastic pyrolysis involve the decomposition of polymer materials at high temperatures in an oxygen-free or low-oxygen environment. However, their molecular structures and chemical bond characteristics differ, leading to differences in their pyrolysis mechanisms.
Plastic Waste Pyrolysis Principle
Plastics have a relatively regular molecular structure, and their chemical bond energies are relatively uniform. During pyrolysis, plastic molecules decompose primarily through the cleavage of C-C and C-H bonds. Depending on the location of bond cleavage within the macromolecule, plastic pyrolysis can be categorized as depolymerization, random pyrolysis, and intermediate pyrolysis.
Depolymerization-type plastics undergo thermal pyrolysis, where the polymer dissociates, primarily severing the chemical bonds between individual molecules, resulting in almost 100% degradation into monomers.
Random pyrolysis-type plastics undergo random cleavage of chemical bonds upon heating, producing a molecular compound composed of a certain number of carbon and hydrogen atoms.
Most plastics undergo intermediate pyrolysis, combining characteristics of both types.
Tyre Rubber Pyrolysis Principle
Rubbers have a more complex molecular structure, typically containing numerous double bonds and crosslinks. These double bonds and crosslinks enhance the interactions between rubber molecules and result in an uneven distribution of chemical bond energies.
During pyrolysis, rubber molecules not only have to overcome the bond energy of C-C and C-H bonds, but also have to break down double bonds and crosslinks. Therefore, the pyrolysis process is relatively complex.
Rubber pyrolysis first undergoes two stages: gas-phase pyrolysis and solid-phase pyrolysis. These reactions break down the chemical bonds within the rubber molecules, breaking them into smaller molecules. These small molecules then react further under high temperature and oxygen conditions to form even smaller compounds.
Differences in Pyrolysis Processes
Pyrolysis processes differ significantly between rubber and plastic. These differences primarily occur in pyrolysis temperature, time, and equipment.
Pyrolysis Temperature
Regarding pyrolysis temperature, plastics typically decompose between 350°C and 600°C, depending on the type of plastic and the pyrolysis process. For example, polyethylene (PE) decomposes primarily after 400°C. This is because it lacks reactive side chains and has high thermal stability.
Rubber decomposes at temperatures generally between 600°C and 800°C, higher than the plastic decomposition temperature range. This is because the double bonds and crosslinks in rubber molecules require higher temperatures to break down, allowing the pyrolysis reaction to proceed.
Pyrolysis Time
Plastics decompose relatively quickly, typically between a few minutes and a few hours. This is because the molecular structure of plastics is relatively simple, allowing chemical bonds to break and reform quickly under suitable temperature conditions.
Due to its complex molecular structure, rubber waste pyrolysis involves multiple reaction stages and takes longer to complete, typically ranging from several to over ten hours.

Pyrolysis Equipment
Both plastic and rubber waste pyrolysis equipment can be used in batch pyrolysis equipment or continuous pyrolysis system. However, due to their different physical properties and pyrolysis characteristics, the pyrolysis equipment requirements differ.
Plastics are generally softer and more fluid, making them prone to agglomeration during pyrolysis. Therefore, plastic pyrolysis requires higher stirring and heat transfer performance in the plastic pyrolysis plant.
Rubber, on the other hand, is harder and more elastic. It requires pretreatment before pyrolysis, such as crushing and pulverization, to improve pyrolysis efficiency.
Furthermore, the waste pyrolysis process of rubber produces a large amount of gas and tar, placing more stringent requirements on the gas treatment and tar recovery systems of the tyre pyrolysis plant.
Differences in Pyrolysis Products
The pyrolysis products of rubber and plastics also differ significantly in type, composition, and uses.
Synthesis Gas
Regarding pyrolysis gas, the gases produced by waste plastic pyrolysis are primarily mixed light hydrocarbons, such as methane (CH4), ethane (C2H6), ethylene (C2H4), and propane (C3H8). These gases can be used as fuel gas or chemical feedstocks.
In addition to hydrocarbon gases, rubber waste pyrolysis gas also contains significant amounts of hydrogen (H2) and methane (CH4). Hydrogen has a high calorific value and can be used as a clean energy source. Methane, a major component of natural gas, also has significant energy value. Gases produced by the pyrolysis of natural rubber include methane, ethane, ethylene, propylene, hydrogen, butadiene, and others.

Pyrolysis Oil
Pyrolysis oil is a key product of the pyrolysis of rubber and plastics, and they differ in composition and properties.
Plastic pyrolysis oil is primarily composed of aliphatic compounds, such as alkanes and alkenes of various chain lengths. Its properties are similar to fuel oil obtained from petroleum refining and can be used as an engine fuel or chemical raw material.
Rubber pyrolysis oil contains a high concentration of aromatic compounds, such as benzene, toluene, and xylene. These aromatic compounds are highly chemically active and can be used in the synthesis of polymer materials such as rubber, plastics, and fibers, as well as as solvents and chemical raw materials. The liquid products produced during the pyrolysis of scrap tires contain highly valuable compounds, such as benzene, toluene, and xylene.
Carbon Black
Carbon black is the solid product remaining after the pyrolysis of rubber and plastics.
The main components of the solid product from the pyrolysis of plastics are carbon black and some inorganic impurities. The carbon black content is relatively low, and the quality is inferior to that of charcoal from rubber pyrolysis. Carbon black from plastic pyrolysis can be used to produce activated carbon, rubber reinforcing agents, and other applications.
The carbon black produced from rubber pyrolysis has a higher content and is of higher quality, with properties similar to those of traditional carbon black. Carbon black from tire pyrolysis can be used as a filler in rubber products, replacing some traditional carbon black and reducing production costs. It can also be used to produce activated carbon for adsorption and purification applications.